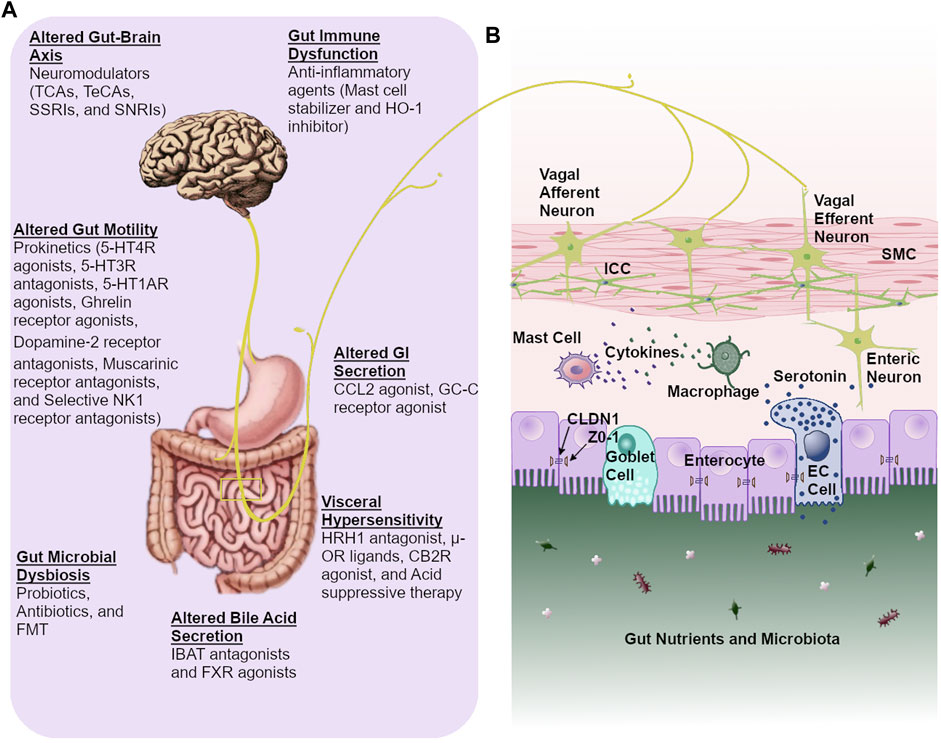
Frontiers | Current Treatment Options and Therapeutic Insights for Gastrointestinal Dysmotility and Functional Gastrointestinal Disorders | Pharmacology

Pharmacological agents currently in clinical trials for disorders in neurogastroenterology. - Abstract - Europe PMC

Velusetrag accelerates gastric emptying in subjects with gastroparesis: a multicentre, double‐blind, randomised, placebo‐controlled, phase 2 study - Kuo - 2021 - Alimentary Pharmacology & Therapeutics - Wiley Online Library

Phase 2 Study of Velusetrag in Diabetic or Idiopathic Gastroparesis | Clinical Research Trial Listing ( Gastroparesis ) ( NCT01718938 )

Theravance Biopharma Receives FDA Fast Track Designation for Velusetrag (TD-5108) for Idiopathic and Diabetic Gastroparesis

Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis | Gut

Theravance Biopharma Announces Positive Top-Line Results from Phase 2b Study of Velusetrag (TD-5108) in Patients with Gastroparesis

Efficacy and Safety of Tradipitant in Patients With Diabetic and Idiopathic Gastroparesis in a Randomized, Placebo-Controlled Trial - ScienceDirect

Clinical trial: the efficacy and tolerability of velusetrag, a selective 5‐HT4 agonist with high intrinsic activity, in chronic idiopathic constipation – a 4‐week, randomized, double‐blind, placebo‐controlled, dose–response study - Goldberg - 2010 -

Clinical trial: the efficacy and tolerability of velusetrag, a selective 5‐HT4 agonist with high intrinsic activity, in chronic idiopathic constipation – a 4‐week, randomized, double‐blind, placebo‐controlled, dose–response study - Goldberg - 2010 -

Theravance Biopharma Announces Positive Top-Line Results from Phase 2b Study of Velusetrag (TD-5108) in Patients with Gastroparesis

Efficacy of drugs in chronic idiopathic constipation: a systematic review and network meta-analysis - The Lancet Gastroenterology & Hepatology